Will form ammonia, water and calcium chloride.
Fehling's Test. In this test the presence of aldehydes but not ketones is detected by reduction of the deep blue solution of copper(II) to a red precipitate of insoluble copper oxide. The test is commonly used for reducing sugars but is known to be NOT specific for aldehydes.
Glass ,plastic, mirrors
Different people or groups can have differing opinions as to what is best. Unless they say top selling which is based on sales, the rest is all opinion and can vary. And even if they top selling they may quantify it. Sometimes with moves you will hear "America's number one comedy" when it is the … Read more
For books? Or what you talkin bout Willis?
Atom : Individual particle made up of electrons and protons and neutrons
Molecule : Electrically neutral group of two or more atoms.
42%. Or
Ask your parents, math teacher, tutors and student groups. Of course, your math book 📚has examples and you could practice and certainly figure out this answer.
78.09% because I copied from that cheater Echooos.
Cause without Chemistry, you'll be dead. I mean, simply the air you breathe is a part of it.
Hey Otis, let's add national answer-an-old-question day to the calendar---it's proving to be worthwhiule. .
In general, nitrogen is unreactive at standard temperature and pressure.
To understand the abundance of N in the atmosphere, it is useful to compare it to O (the next most abundant element in the atmosphere). Compared to O, N is 4 times as abundant in the atmosphere. However, we must also consider the relative abundances of O and N over the entire Earth … Read more
do you mean latent as quality or state, or as biology latent
The purpose of a blast furnace is to chemically reduce and physically convert iron oxides into liquid iron called "hot metal". The blast furnace is a huge, steel stack lined with refractory brick, where iron ore, coke and limestone are dumped into the top, and preheated air is blown into the bottom. The hot blast … Read more
High thermal and electrical conductivity.
Luster and high reflectivity.
Malleability and ductility. They can be beaten or shaped without fracture.
Variability of mechanical strengths (ranging from soft alkali metals to Tungsten, which is hard).
Reference: http://chemistry.tutorvista.com/physical-chemistry/metallic-bonding.htmlHow about Antennapedia Peptide? Can it be used as a reference?
The human body is obtusely complicated entity with many mechanisms that are influenced if not controlled by chemicals in many ways.
One of the more interesting exercises in a chemistry teaching lab is showing the real manifestation of water's polarity.
Turn on a faucet and let a stream of water flow while putting a magnet next to the stream. Depending on the polarity one will see the stream attracted to or repeled by the magnet.
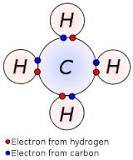
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.
Covalent bond - Wikipedia, the free encyclopedia